From July 8-11, 2019, the FDA performed an inspection at California Cereal Products’ dry cereal manufacturing facility in Macon, GA. The inspection resulted in the Agency issuing a Warning Letter to the company. Although there were no label compliance issues to highlight from this one, the serious cGMP violations were almost TNTC (too numerous to count).
Four major observations of violations made it into the Warning Letter:
- They did not take effective measures to exclude pests from their food plant to protect against contamination of food. It’s a little hard to chart, as in many cases the pests were TNTC – but we tried. For reference, four live American cockroaches were spotted.
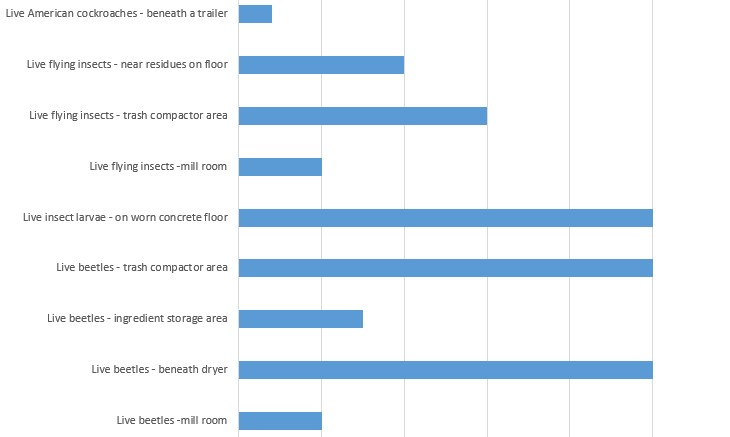
- Their plant was not constructed in such a manner that drip and condensate from fixtures, ducts, and pipes does not contaminate food, food-contact surfaces, or food packaging materials.
- Their equipment and utensils were not adequately maintained to protect against allergen cross-contamination.
- Their plant floors were not constructed in a manner that they could be adequately cleaned.
Exterior of inspected facility.
To read the letter in full, click HERE.
If anything in the letter sounds familiar from your facility, or you would just like to be better prepared for your own FDA inspection when the time comes, let us know. In addition to label compliance reviews, we do facility audits and consulting on following cGMPs. Contact us today to discuss your needs and get a free quote.

